In June 2024, a significant development in the field of precision medicine was reported: researchers have achieved a 95% reduction in attacks of hereditary angioedema (HAE) through the use of CRISPR-Cas9 gene editing technology. The study, conducted by Vertex Pharmaceuticals in collaboration with CRISPR Therapeutics, represents one of the most compelling demonstrations to date of gene editing as a viable therapeutic strategy for chronic systemic genetic diseases.
Hereditary angioedema is a rare autosomal dominant disorder characterized by recurrent and unpredictable episodes of severe swelling, often affecting the face, gastrointestinal tract, and upper airways. The condition arises due to mutations in the SERPING1 gene, which encodes the C1 esterase inhibitor, a protein essential for regulating vascular permeability and inflammation. Conventional therapeutic approaches for HAE typically involve regular intravenous or subcutaneous administration of C1 inhibitor concentrate or kallikrein inhibitors. While these treatments have been effective in reducing the frequency and severity of attacks, they remain palliative rather than curative and impose a substantial financial burden on healthcare systems, with annual costs often exceeding half a million US dollars per patient.
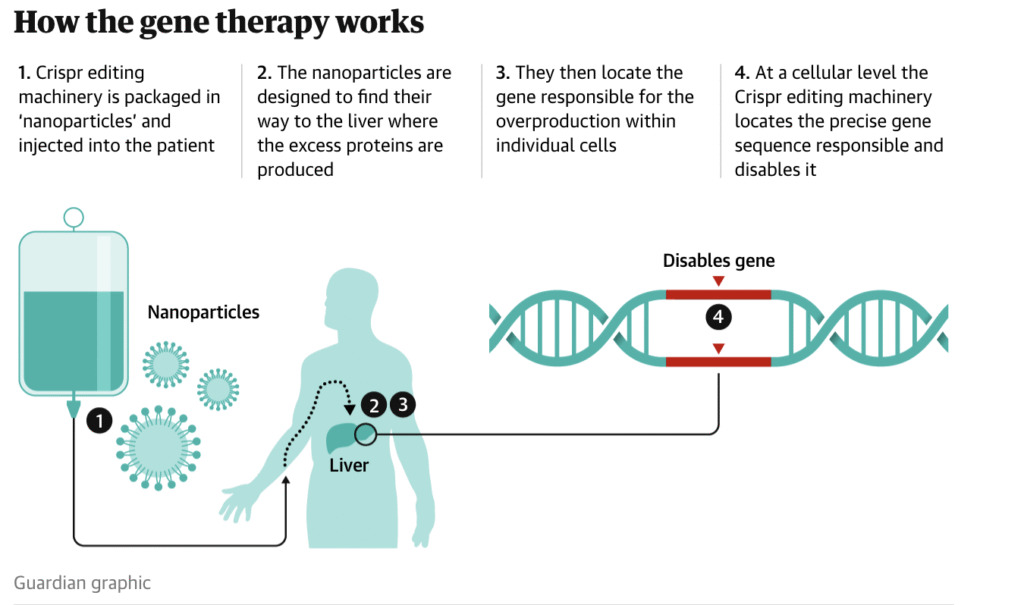
The newly developed CRISPR-based therapeutic approach directly targets hepatocytes, the primary site of C1 inhibitor production. Using the CRISPR-Cas9 system, researchers introduced precise edits into the SERPING1 gene to restore normal expression levels of the protein. In the initial phase of the clinical trial, ten patients received a single infusion of the gene-editing treatment. The results were notable: the frequency of angioedema attacks decreased by an average of 95 percent across participants. Moreover, the safety profile was favorable, with no serious adverse events observed during or after treatment. In several cases, patients remained symptom-free for more than a year following administration, suggesting that the intervention may produce durable, long-lasting effects.
This study is particularly significant because it marks the first successful application of CRISPR technology in treating a chronic, multi-organ genetic disorder. Previous clinical trials of CRISPR-based therapies have primarily focused on hematologic conditions such as sickle cell disease and beta-thalassemia, where gene editing occurs ex vivo and targets relatively accessible stem cell populations. In contrast, the current trial demonstrates the feasibility of in vivo gene editing in solid organs, thereby broadening the potential applications of the technology.
The implications of this breakthrough extend beyond HAE. The successful targeting and editing of liver cells using CRISPR suggest that similar strategies could be adapted for other monogenic disorders with hepatic expression profiles, including hemophilia, alpha-1 antitrypsin deficiency, and certain metabolic diseases. Furthermore, as gene editing platforms become more refined, it is plausible that such approaches may be extended to polygenic and neurodegenerative diseases, including forms of Alzheimer’s and Parkinson’s disease, that have hitherto eluded curative interventions.
Looking ahead, the therapy is expected to enter Phase 3 clinical trials in 2025. If subsequent trials confirm the efficacy and safety observed thus far, regulatory submissions to the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) could follow by 2026. Additionally, preclinical research is already underway to expand the platform’s reach to other genetic conditions.
In sum, this development marks a pivotal moment in the evolution of genetic therapeutics. The ability to address the underlying genetic etiology of a complex, systemic disorder through a single-dose intervention not only enhances the therapeutic arsenal available to clinicians but also underscores the promise of CRISPR as a cornerstone of future medical practice. As regulatory pathways adapt to these technological advancements, and as long-term safety data accumulate, the prospect of eradicating inherited diseases at their genetic root is becoming increasingly realistic.